Abstract
Introduction Tyrosine kinase inhibitors (TKIs) have facilitated the development of low intensity induction regimens for Philadelphia-chromosome positive (Ph+) ALL. Whether the use of a low intensity induction regimen prior to allogeneic hematopoietic stem cell transplant (HSCT) impacts post-HSCT outcomes is not known. We studied patients with Ph+ ALL receiving HSCT in CR1 at our center to assess the impact of induction treatment intensity on post-HSCT outcomes.
Methods We identified consecutive adult patients with Ph+ ALL who underwent HSCT in CR1 between January 1, 2010 and April 31, 2021. Induction therapy was classified as low intensity (TKI, corticosteroid, with or without vincristine) or high intensity (all others). Pre-HSCT measurable residual disease negative (MRDneg) status was determined by BCR::ABL1 RT-PCR and/or via multi-parameter flow cytometry with sensitivity of at least 4.7 and 4 logs respectively. Comparisons between variables were assessed using the Fisher exact (categorical) or Wilcoxon rank-sum (continuous) tests. Overall survival (OS) was defined as time from HSCT to death. Disease-free survival (DFS) was defined as time from HSCT to hematologic relapse or death. The OS and DFS analyses were performed using Cox proportional hazards regression, and differences were assessed using the log rank test. The cumulative incidence of relapse (CIR) was compared between the high versus low intensity induction therapy groups from the time of transplant, with non-relapse mortality (NRM) a competing event.
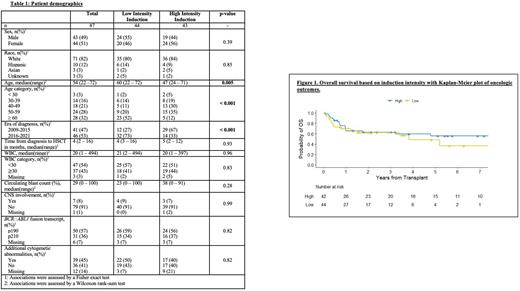
Results A total of 87 patients with Ph+ ALL transplanted in CR1 were identified. Patients in the low intensity induction cohort (n=44, 51%) were older (median 60 vs 47 yrs, p<0.01) and more frequently diagnosed after 2015 (73% vs 33%, p<0.01) compared to patients in the high intensity cohort (n=43, 49%). Clinical and pathologic characteristics including initial white blood cell count, presence of central nervous system disease, BCR::ABL1 transcript type (p190 vs. p210), and presence of additional cytogenetic abnormalities did not differ between groups. There was no difference between the low and high induction intensity cohorts in the proportion of patients requiring a salvage regimen to achieve CR1 (14% vs. 16%, p=0.77). Following induction, molecular MRDneg status was similar in the low and high intensity cohorts (54% and 52% respectively, p=0.99). Most patients proceeded directly to alloHSCT after their initial treatment regimen with similar time to alloHSCT from Ph+ ALL diagnosis in both low and high intensity groups (4 vs. 5 months, p=0.93). Patients receiving low intensity induction were numerically more likely to receive a reduced intensity conditioning (RIC) HSCT (39% vs. 19%, p=0.06). At a median follow-up of 21 months from HSCT, there was no difference between low and high intensity induction with respect to 2-yr DFS (58% vs. 56%, p=0.58), 2-yr OS (62% vs. 63%, p=0.46), 2-yr CIR (9% vs. 17%, p=0.78), and 2yr NRM (33% vs. 29%, p=0.54). A comparison among the low intensity subgroup receiving myeloablative conditioning (MAC) vs. RIC showed no statistically significant difference in OS (HR, 1.02; 95% CI, 0.41-2.54; p=0.97). We segmented patients by pre-HSCT induction intensity and transplant conditioning intensity in the following categories: high intensity induction with MAC HSCT, high intensity induction with RIC HSCT, low intensity induction with MAC HSCT, and low intensity induction with RIC HSCT. Log-rank tests across the four groups showed no significant difference in 2-year DFS (p=0.40), 2-year OS (p=0.19), 2-year CIR (p=0.21), and 2-year NRM (p=0.76).
Conclusion Among adult Ph+ ALL patients transplanted in CR1, the intensity of initial induction regimen does not appear to impact post-HSCT outcomes supporting the use of low intensity induction regimens prior to curative HSCT.
Disclosures
DeAngelo:Glycomimetics: Research Funding; Amgen: Consultancy; Agios: Consultancy; Autolus: Consultancy; Forty-Seven: Consultancy; Incyte: Consultancy; Blueprint Medicines Corporation: Consultancy; Abbvie: Research Funding; Pfizer: Consultancy; Shire: Consultancy; Takeda: Consultancy; Novartis: Consultancy, Research Funding; Jazz Pharmaceuticals: Consultancy. Stevenson:Novartis: Current Employment. Neuberg:Madrigal Pharmaceuticals: Current equity holder in private company. Winer:Curis: Consultancy; Takeda Pharmaceuticals: Consultancy; Abbvie: Consultancy; Novartis, Jazz Pharmaceuticals, Pfizer, Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees. Garcia:AbbVie, Astellas, and Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Advisory Board ; AbbVie: Research Funding; AbbVie, Genentech, AstraZeneca, Prelude and Pfizer: Other: Clinical trial support (institutional) , Research Funding. Kim:Multiple Myeloma Research Foundation: Research Funding; LabCorp: Consultancy. Stone:Aprea: Consultancy; Syndax: Consultancy; OncoNova: Consultancy; Boston Pharmaceuticals: Consultancy; Novartis: Consultancy; Syntrix: Consultancy; Epizyme: Consultancy; Foghorn Therapeutics: Consultancy; Apteva: Consultancy; Janssen: Consultancy; Innate: Consultancy; Actinium: Consultancy; Abbvie: Consultancy, Research Funding; Takeda: Consultancy; GSK: Consultancy; Gemoab: Consultancy; BMS: Consultancy; Elevate Bio: Consultancy; Astellas: Consultancy; Arog: Consultancy, Research Funding; BerGenBio: Consultancy; Syros: Consultancy; Kura Oncology: Consultancy; Jazz: Consultancy. Ho:Jazz: Research Funding; Omeros: Consultancy; Allovir: Consultancy; Alexion: Consultancy. Luskin:Pfizer: Honoraria; Abbvie: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal